YOUR IMMUNE SYSTEM
All living organisms are continuously exposed to substances that are capable of causing them harm. Most organisms protect themselves against such substances in more than one way — with physical barriers, for example, or with chemicals that repel or kill invaders. Animals with backbones, called vertebrates, have these types of general protective mechanisms, but they also have a more advanced protective system called the immune system. The immune system is a complex network of organs containing cells that recognize foreign substances in the body and destroy them. It protects vertebrates against pathogens, or infectious agents, such as viruses, bacteria, fungi, and other parasites. The human immune system is the most complex.
Although there are many potentially harmful pathogens, no pathogen can invade or attack all organisms because a pathogen's ability to cause harm requires a susceptible victim, and not all organisms are susceptible to the same pathogens. For instance, the virus that causes AIDS in humans does not infect animals such as dogs, cats, and mice. Similarly, humans are not susceptible to the viruses that cause canine distemper, feline leukemia, and mouse pox.
Two Kinds of Immunity
All animals possess a primitive system of defense against the pathogens to which they are susceptible. This defense is called innate, or natural, immunity and includes two parts. One part, called humoral innate immunity, involves a variety of substances found in the humors, or body fluids. These substances interfere with the growth of pathogens or clump them together so that they can be eliminated from the body. The other part, called cellular innate immunity, is carried out by cells called phagocytes that ingest and degrade, or “eat'' pathogens and by so-called natural killer cells that destroy certain cancerous cells. Innate immunity is nonspecific — that is, it is not directed against specific invaders but against any pathogens that enter the body.
Only vertebrates have an additional and more sophisticated system of defense mechanisms, called adaptive immunity, that can recognize and destroy specific substances. The defensive reaction of the adaptive immune system is called the immune response. Any substance capable of generating such a response is called an antigen, or immunogen. Antigens are not the foreign microorganisms and tissues themselves; they are substances — such as toxins or enzymes — in the microorganisms or tissues that the immune system considers foreign. Immune responses are normally directed against the antigen that provoked them and are said to be antigen-specific. Specificity is one of the two properties that distinguish adaptive immunity from innate immunity. The other is called immunologic memory. Immunologic memory is the ability of the adaptive immune system to mount a stronger and more effective immune response against an antigen after its first encounter with that antigen, leaving the organism better able to resist it in the future.
Adaptive immunity works with innate immunity to provide vertebrates with a heightened resistance to microorganisms, parasites, and other intruders that could harm them. However, adaptive immunity is also responsible for allergic reactions and for the rejection of transplanted tissue, which it may mistake for a harmful foreign invader.
Lymphocytes — Heart of the Immune System
Lymphocytes — a class of white blood cells — are the principal active components of the adaptive immune system. The other components are antigen-presenting cells, which trap antigens and bring them to the attention of lymphocytes so that thev can mount their attack.
How lymphocytes recognize antigens
A lymphocyte is different from all other cells in the body because it has about 100,000 identical receptors on its cellular membrane that enable it to recognize one specific antigen. The receptors are proteins containing grooves that fit into patterns forrned by the atoms of the antigen molecule — somewhat like a key fitting into a lock — so that the lymphocyte can bind to the antigen. There are more than 10 million different types of grooves in the lymphocytes of the human immune system.
When an antigen invades the body, normally only those lymphocytes with receptors that fit the contours of that particular antigen take part in the immune response. When they do, so-called daughter cells are generated that have receptors identical to those found on the original lymphocytes. The result is a family of lymphocytes, called a lymphocyte clone. with identical antigen-specific receptors.
A clone continues to grow after lymphocytes first encounter an antigen so that, if the same type of antigen invades the body a second time, there will be many more lymphocytes specific for that antigen ready to meet the invader This is a crucial component of immunologic memory.
How lymphocytes are made
Like all blood cells, lymphocytes are made from stem cells in the bone marrow (see Blood, "Composition"). (In fetuses, or unborn offspring, lymphocytes are made in the liver.) Lymphocytes then undergo a second stage of development, or processing, in which they acquire their antigen-specific receptors. By chance, some lymphocytes are created with receptors that happen to be specific to normal, healthy components of the body. Fortunately, a healthy immune system purges itself of these lymphocytes, leaving only lymphocytes that ignore normal body components but react to foreign intruders. If this purging process is not completely successful, the result is an autoimmune (literally "self-immune") disease in which the immune system attacks normal components of the body as though they were foreign antigens, destroying healthy molecules, cells, or tissues.
Some lymphocytes are processed in the bone marrow and then migrate to other areas of the body — specifically the lymphoid organs (see Lymphatic System). These lymphocytes are called B lymphocytes, or B cells (for bone-marrow-derived cells). Other lymphocytes move from the bone marrow and are processed in the thymus, a pyramid-shaped lymphoid organ located immediately beneath the breastbone at the level of the heart. These lymphocytes are called T lymphocytes, or T cells (for thymus-derived cells).
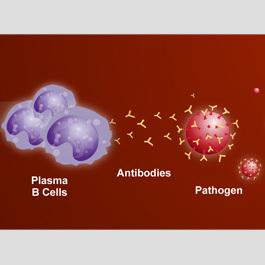
These two types of lymphocytes — cells and T cells — play different roles in the immune response, though they may act together and influence one another's functions. The part of the immune response that involves B cells is often called humoral immunity because it takes place in the body fluids. The part involving T cells is called cellular immunity because it takes place directly between the T cells and the antigens. This distinction is misleading, however, because, strictly speaking, all adaptive immune responses are cellular — that is, they are all initiated by cells (the lymphocytes) reacting to antigens. B cells may initiate an immune response, but the triggering antigens are actually eliminated by soluble products that the B cells release into the blood and other body fluids. These products are called antibodies and belong to a special group of blood proteins called immunoglobulins When a B cell is stimulated by an antigen that it encounters in the body fluids, it transforms, with the aid of a type of T cell called a helper T cell (see "T cells"), into a larger cell called a blast cell. The blast cell begins to divide rapidly, forming a clone of identical cells.
Some of these transform further into plasma cells — in essence, antibody-producing factones. These plasma cells produce a single type of antigen-specific antibody at a rate of about 2,000 antibodies per second. The antibodies then circulate through the body fluids, attacking the triggering antigen.
Antibodies attack antigens by binding to them. Some antibodies attach themselves to invading microorganisms and render them immobile or prevent them from penetrating body cells. In other cases, the antibodies act together with a group of blood proteins, collectively called the complement system, that consists of at least 30 different components. In such cases, antibodies coat the antigen and make it subject to a chemical chain reaction with the complement proteins. The complement reaction either can cause the invader to burst or can attract scavenger cells that "eat" the invader.
Not all of the cells from the clone formed from the original B cell transform into antibody-producing plasma cells; some serve as so-called memory cells. These closely resemble the original B cell, but they can respond more quickly to a second invasion by the same antigen than can the original cell. T cells. There are two major classes of T cells produced in the thymus: helper T cells and cytotoxic, or killer, T cells. Helper T cells secrete molecules called interleukins (abbreviated IL) that promote the growth of both B and T cells. The interleukins that are secreted by lymphocytes are also called lymphokines. The interleukins that are secreted by other kinds of blood cells called monocytes and macrophages are called monokines. Some ten different interleukins are known: IL-1, IL-2, IL-3, IL-4, IL-5, IL-6, IL-7, interferon, lymphotoxin, and tumor necrosis factor. Each interleukin has complex biological effects.
Cytotoxic T cells destroy cells infected with viruses and other pathogens and may also destroy cancerous cells. Cytotoxic T cells are also called suppressor lymphocytes because they regulate immune responses by suppressing the function of helper cells so that the immune svstem is active onlv when necessary.
The receptors of T cells are different from those of B cells because they are "trained" to recognize fragments of antigens that have been combined with a set of molecules found on the surfaces of all the body's cells. These molecules are called MHC molecules (for major histocompatibility complex). As T cells circulate through the body, they scan the surfaces of body cells for the presence of foreign antigens that have been picked up by the MHC molecules. This function is sometimes called immune surveillance.
Immune Response
When an antigen enters the body, it may be partly neutralized by components of the innate immune system. It may be attacked by phagocytes or by preformed antibodies that act together with the complement system. Often, however, the lymphocytes of the adaptive immune system are brought into play.
The human immune system contains approximately l trillion T cells and l trillion B cells, located in the lymphoid organs and in the blood, plus approximately 10 billion antigen-presenting cells located in the lymphoid organs. To maximize the chances of encountering antigens wherever they may invade the body, lymphocytes continually circulate between the blood and certain lymphoid tissues. A given lymphocyte spends an average of 30 minutes per day in the blood and recirculates about 50 times per day between the blood and lymphoid tissues.
If lymphocytes encounter an antigen trapped by the antigen-presenting cells of the lymphoid organs, lymphocytes with receptors specific to that antigen stop their migration and settle to mount an immune response locally. As these lymphocytes accumulate in the affected lymphoid tissue, the tissue often becomes enlarged — for example, the lymph nodes in the groin become enlarged if there is an infection in the thigh area.
Antigen-presenting cells degrade antigens and often eliminate them without the help of lymphocytes. If there are too many antigens for them to handle alone, however, the antigen-presenting cells secrete IL- 1 and display fragments of the antigens (combined with MHC molecules) to alert the helper T cells. The IL-1 facilitates the responsiveness of T and B cells to antigens and, if released in large amounts (as it is in the course of infections), can also cause fever and drowsiness. Helper T cells that encounter IL- 1 and fragments of antigens transform into cells called lymphoblasts, which then secrete a variety of interleukins that are essential to the success of the immune response. The IL-2 produced by helper T cells promotes the growth of cytotoxic T cells, which may be necessary to destroy tumorous cells or cells infected with viruses. The IL-3 increases the production of blood cells in the bone marrow and thus helps to maintain an adequate supply of the lymphocytes and lymphocyte products necessary to fight infections. Helper T cells also secrete interleukins that act on B cells, stimulating them to divide and to transform into antibody-secreting plasma cells. The antibodies then perform their part of the immune function.
The process of inducing an immune response is called immunization. It may be either natural — through infection by a pathogen — or artificial — through the use of serums or vaccines. The heightened resistance acquired when the body responds to infection is called active immunity. Passive immunity results when the antibodies from an actively immunized individual are transferred to a second, nonimmune subject. Active immunization, whether natural or artificial, is longer-lasting than is passive immunization because it takes advantage of immunologic memory.
Monoclonal Antibodies
Scientists can now produce antibody-secreting cells in the laboratorv by a method known as the hybridoma technique. Hybridomas are hybrid cells made by fusing a cancerous, or rapidly reproducing, plasma cell and a normal antibody-producing plasma cell obtained from an animal immunized with a particular antigen. The hybridoma cell can produce large amounts of identical antibodies — called monoclonal, or hybridoma, antibodies — which have widespread applications in medicine and biology.
BOOST YOUR IMMUNE SYSTEM